Moreover, I find the labeling of ascorbic acid (AA) as a vitamin misleading.
Index:
- Ascorbate Natural History
- Ascorbic acid route administration
- Toxicity
- Human Evidence on Intravenous|Intramuscular Vitamin C
- N=1 (or several) Testing
- Conclusion
Ascorbate Natural History
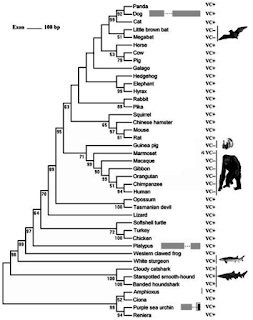 |
| Source: Figure 1 from Conserved or Lost: Molecular Evolution of the KeyGene GULO in Vertebrate Vitamin C Biosynthesis (adapted) |
-
It seems that ascorbic acid production is ancient. It has been hypothesized that eukaryotes evolved the enzyme now used in animals (GULO gene) early on, giving rise to a related one in fungi (some synthesize the ascorbate analogue D-erythroascorbate) and new ones on plants giving rise to ascorbate production without hydrogen peroxide byproduct due to GULO.
From Biosynthesis of Ascorbic Acid in Plants: A Renaissance:Comparisons between the amino acid sequences of the aldonolactone dehydrogenases (green plants)/oxidases (rat) that produce ascorbate or erythroascorbate (yeast) show 26 to 31% identity.
From Evolution of alternative biosynthetic pathways for vitamin C following plastid acquisition in photosynthetic eukaryotes:Here we present molecular and biochemical evidence demonstrating that GULO was functionally replaced with GLDH in photosynthetic eukaryote lineages following plastid acquisition. GULO has therefore been lost repeatedly throughout eukaryote evolution. The formation of the alternative biosynthetic pathways in photosynthetic eukaryotes uncoupled ascorbate synthesis from hydrogen peroxide production and likely contributed to the rise of ascorbate as a major photoprotective antioxidant.
- Nonteleost fishes synthesize ascorbate in their kidneys (some even in their brains) so it isn't a vitamin for them. Nevertheless, there is some effects of supplementation of ascorbic acid under stress.
From Dietary ascorbic acid may be necessary for enhancing the immune response in Siberian sturgeon (Acipenser baerii), a species capable of ascorbic acid biosynthesis (2006):There were no significant differences in renal l-gulono-1, 4-lactone oxidase (GLO) activity between dietary and LPS treatments, but fish exposed to LPS without dietary AA demonstrated an inter-organ transfer of AA from posterior kidney to liver. Our results indicate that dietary AA may be conditionally necessary for Siberian sturgeon to achieve optimal immune response, particularly in early developmental stages, information imperative to developing successful aquaculture programs for sturgeon species.
Many papers reported that supplementation of dietary vitamin C to the animals with AA biosynthesis capability can raise up the animal's anti-stress ability and immune functions (Pardue and Thaxton, 1985, Bains, 1996, Li et al., 2001, Zhou et al., 2002 and Zhou et al., 2005).
- Teleost fishes doesn't make ascorbate so it is a vitamin for them. Nevertheless, we have some special effects at high doses.
From Optimization of dietary vitamin C in fish and crustacean larvae: a review (1997) (my bolds):In studies with European sea basss using diets supplemented with high AA concentrations, positive effects on stress resistance have been observed in salinity stress tests (Merchie et al., 1995b). Also for turbot, cumulative mortalities after challenge with Vibrio anguillarum increased up to 50% for the control, while only 40% mortality occurred in the groups fed vitamin C-enriched diets, providing evidence for an immunostimulatory effect of high AA doses. This lower stress sensitivity was confirmed also for African catfish (Merchie et al., 1995b, 1997b).
- It seems kidney production was conserved by amphibians, reptiles and some birds while been transfered to the liver of most mammals and other birds. Nevertheless, supplementation of ascorbic acid under stress still seems beneficial.
From Ascorbic acid decreases heat shock protein 70 and plasma corticosterone response in broilers (Gallus gallus domesticus) subjected to cyclic heat stress (2004):Under stressful environments, physiological requirements for AA may exceed the AA synthesizing ability of chickens (Pardue and Thaxton, 1986). These authors cited several studies that suggest beneficial effects due to heat stress amelioration and acclimation associated with AA supplementation.
From Vitamin C and infections in animals by Harri Hemilä (excerpt of his dissertation Do vitamins C and E affect respiratory infections?):Rawal et al. (1974) reported that survival of mice infected with Pseudomonas aeruginosa was increased by vitamin C supplementation in a dose-dependent fashion. Senatuite and Biziulevicius (1986) reported that in rats infected with Trichinella spiralis the average number of muscle larvae after 3 weeks was 40% lower in the vitamin C administered group. Chaiyotwittayakun et al. (2002) induced mastitis in cows using intramammary infusion of endotoxin, and vitamin C reduced the fall in milk production caused by endotoxin.
Dr. Hemilä has tackled also experiments with tetanus toxin on animals (I will rewrite my comments about it and the one on humans —first and second— in another blog). Actually it seems that liver ascorbic acid production is increased under stress at least in mammals. From Eight Decades of Scurvy by Irwin Stone:This liver metabolite, ascorbate, is produced in an unstressed goat for instance, at the rate of about 13,000 mg per day per 150 pounds body weight (Chatterjee, 1973). A mammalian feedback mechanism increases this daily ascorbate production many fold under stress (Subramanian et al., 1973).
It seems to happen in ill cats and dogs too.
It seems that these animals are also prone to increase the value of their urine. From Metabolic interactions between l-ascorbic acid and drugs by Conney et al. (1961):The urinary excretion of ascorbic acid increased from control values of about 0.3 mg. per day to values of 17 mg. per day by 6 days after the dose.
- Some species of birds and mammals have lost the capacity of ascorbic acid production, so technically it has become a vitamin for us too. Nevertheless, it seems it has some special effects at high doses.
There has been some research on the effectiveness of enough intramuscular ascorbic acid (100mg/kg twice a day for 7 days) on rabies (guinea pigs) by Dr. Banič (Prevention of rabies by vitamin C, full text) that is still unrefuted: gross estimation of the probability of 17 or less of the 48 guinea pigs in the treatment group dying and 35 or more of the 50 controls dying supposing it being completely by chance —supposing probability of a death being 52/98— is 0.01%.
We humans (perhaps some but not all Haplorrhini primates) have developed unique adaptations such as not burning it into carbon dioxide and water unlike guinea pigs. Our kidneys work hard to minimize its loses in urine (it seems an ubiquitous mechanism at least in mammals) whenever its concentration drops below a certain threshold. This threshold varies from person to person mostly between 0.9 and 2.0mg/dl.
Reading late Irving Stone's state of the art (at the time) review (its credentials are immaterial since he didn't do the experiments himself, it could do its review incomplete though... good luck finding a more thorough one) on vitamin C is a must.
All of these data coupled with both its low toxicity and clinical experience by Drs. Klenner, Cathcart and Levy point toward ascorbic acid supplementation at high doses as a therapy to be considered plausible on humans and so to be refuted, not ignored.
Ascorbic acid route administration
Of course achieving an equivalent serum value should produce equivalent outcomes whichever the route of administration. But.
 |
| Peak plasma concentration on oral (circles) versus intravenous (triangles) delivery Source: Vitamin C Pharmacokinetics: Implications for Oral and Intravenous Use |
Moreover. From Pharmacokinetics of Vitamin C: insights into the oral and intravenous administration of ascorbate:
Model-derived overprediction of plasma ascorbate concentrations with respect to the observed ones in several different literature reports could be the result of incomplete absorption of administered vitamin C at daily doses higher than 200 mg (1.1 mmol). Otherwise, it could also be a result of inappropriate timing of actual measurements.About oral absorption we have for example the paper by Hornig et alter* stating:
A male non-smoking volunteer increased his daily intake of ascorbic acid continuously by ingesting in a single, oral dose 1, 2, 3, 4 and 5 g crystalline ascorbic acid.
The urinary excretion of unmetabolized unlabelled ascorbic acid per day was taken as index for the absorption of ascorbic acid. It decreased from 75% (1 g), 44.0% (2 g), 39% (3g), 28% (4 g) to 20% (5 g) of the ingested ascorbic acid.These data are about a healthy individual. Clinical experience of Dr. Cathcart points toward quite different dynamics under illness.
What's my point? There is no use to mix intravenously|intramuscularly delivered trials with orally delivered ones when assessing ascorbate efficacy.
Finally, there is my concern about the subcutaneous delivery route.
Subcutaneous route and poliomyelitis
Late Dr. Klenner's put the blame of the fail of vitamin C curing poliomyelitis in monkeys on Dr. Sabin unsuccessful replication of Dr. Jungeblut's results. From The Use of Vitamin C as an Antibiotic:
One of the most unfortunate mistakes in all of the research on poliomyelitis was Sabin’s UN-SCIENTIFIC attempt to confirm Jungeblut’s work with vitamin C against the Polio virus in monkeys. Jungeblut in infecting his Rhesus monkeys used the mild ‘droplet method’ and then administered vitamin C by needle in varying amounts up to 400 mgm/day. Even this method did not give him absolute control over the degree of infection that would result. However, his antibiotic (vitamin C) remained relatively constant. With almost infinitesimal amounts, as we at present recognize, he was able to demonstrate in one series that the non-paralytic survivors was six times as great as in the controls. On the other hand, Sabin, in infecting his monkeys did not follow the procedure given by Jungeblut who’s experiments he was attempting to repeat, but instead employed a more forceful method of inoculation which obviously resulted in sickness of maximum severity. Sabin further refused to follow Jungeblut’s suggestion as to the dose of vitamin C to be used. By Sabin’s actual report the amount given was rarely more than 35 per cent of that used by his associate. Sabin makes this significant statement (1939)7, “One monkey was given 400 mgm of vitamin C for one day at the suggestion of Jungeblut who felt that large doses was necessary to effect a change in the course of the disease.” Yet on the basis of Sabin’s work the negative value of vitamin C in the treatment of virus diseases has been for years accepted as final.This has been the core of every critique henceforward to this day by those on an orthomolecular path such as Andrew W. Saul or Steve Hickey. I would like to point out majkinetor's critique:
It was Dr. Jungeblut who found a inverted U-shape response curve as a function of vitamin C dose in his first 1937 paper:
 |
| Source: Table I of Vitamin C Therapy and Prophylaxis in Experimental Polimyelitis showing higher effect with 5mg than with 10-50mg, no effect at all with 100-700mg |
I think that we could blame subcutaneous route (instead of intraperitoneal, intravenous or intramuscular one) while not falsified, since Jungeblut says on the third page of his first 1937 paper:
The doses of vitamin C covered a range from 700 mg. to 5 mg. and were mostly administered by the subcutaneous route.
I would like to know if increasing doses of subcutaneous vitamin C injections could give rise to blocked absorption of it. Dr. Klenner observed "Induration (only when intramuscular injections are given too close to the surface)" so perhaps using the subcutaneous route impeded good absorption of high doses in Dr. Jungeblut's experiment. It would explain the no-effect of higher doses found by Dr. Jungeblut commented on his first paper. If true using subcutaneous route would be the original cause of vitamin C megadoses blackout for more than 70 years already. It wouldn't be posterior failed replication by Dr. Sabin.
Since then I have only found one somewhat similar inverse U-shape response with vitamin C injection using the subcutaneous route for tetanus toxin by Ghosh and Guha in 1938 commented upon by Dr. Hemilä. From their paper: "One m. l. d. of the toxin was injected, and ascorbic acid (100 mg.) in saline (1 c.c.) was injected within one minute after the injection of the toxin, and on a site remote from that of the toxin injection." Using two sites I think that discards intraperitoneal route (used successfully by Dey in rats). Since the minimum lethal dose is defined by subcutaneous route in the tetanus toxin case and they weren't more specific I have to conclude that Gosh and Guha used the subcutaneous route both for the toxin and the vitamin C.
Liposomal vitamin C
 |
| Source: Wikipedia |
One delivery method that differs substantially in the route of absorption is the encapsulation in liposomes. Liposomes are tiny, soluble particles with a lipid bilayer encompassing a substance we want to protect in order to deliver it either directly to cells if we inject them intravenously or to the lymphatic system if we take them orally as pointed out by studentroland.
Oral liposomal vitamin C is absorbed directly to the lymphatic system not longer inside liposomes. In Dr. Levy's words:
Liposomes predominately get taken up by the lymphatic system in the gut, not the portal circulation. There is no significant "one-pass" liver metabolism that takes place with a quality liposome preparation.
On the other hand, the liposomes, especially in the case of those containing vitamin C, rapidly load up the immune cells in the lymphatics of the gut, achieving high intracellular levels of this nutrient.
Colloquially speaking, one could say this "supercharges" the immune system cells.
Those lymphocytes in the thoracic duct and upwards (such as natural killer cells) will be exposed to higher vitamin C concentrations than the one measured in blood. If true it would explain why Dr. Levy gets results with lower doses than predicted by the dynamic flow model (IVC = intravenous vitamin C):
In a nutshell, I found that liposome encapsulated vitamin C, taken orally, was roughly 10 times more effectively clinically in resolving infectious diseases than the IVC.Nevertheless I am unaware of any either animal or human trial using liposomal encapsulated vitamin C.
Toxicity
The only real danger of vitamin C megadosing I have found is for those with a defect in their G6PD enzyme gene.He was also prescribed a course of high dose intravenous ascorbic acid, 40 g three times weekly, supplemented by 20-40 g ascorbic acid daily by mouth. This proceeded uneventfully for about a month with no obvious evidence of either haemolysis or regression of lymphadenopathy. The intravenous dose was increased to 80 g, and next day the patient became breathless and feverish and noticed that his urine was black.As Dr. Humphries wrote:
Hemolysis can occur in the rare disorder called glucose 6 phosphatase dehydrogenase deficiency (G6PD deficiency) if mega doses of vitamin C are given- yet there are cases of even those people tolerating vitamin C when they are deficient. Mind you, there are no drugs in the Physicians Desk Reference (PDR) without far more common risks and definitely more side effects than vitamin C. The risk of hemolysis while taking vitamin C, could be blown out of proportion. “The texts and websites that mention this possible effect often assert that vitamin C can cause problems for G6PD deficient persons when consumed “in high doses.” Search of the medical and scientific literature finds that vitamin C may cause red blood cell rupture (erythrocyte hemolysis) in G6PD deficient adults after massive intravenous infusions (40 to 100 grams within a few hours, or in extremely large oral doses.) There are no reports of this hemolysis problem when oral intake by G6PD deficient persons is less than 6 grams per day in G6PD deficient adults or in healthy adults at any dose.” LINK HEREMoreover, we have the experience of the Riordan Clinic with intravenous vitamin C:
Hemolysis has been reported in patients with G6PD deficiency when given high-dose IVC (Campbell, et al., 1975). The G6PD level should be assessed before beginning IVC. (At the Riordan Clinic, G6PD readings have yielded five cases of abnormally low levels. Subsequent IVC at 25 grams or less showed no hemolysis or adverse effects.)About the possible kidney stones danger on men from some epidemiological studies, first of all I have to point out that they are unlikely to grow in a week of vitamin C treatment. Second, we have late Dr. Cathcart clinical experience:
It is my experience that ascorbic acid probably prevents most kidney stones. I have had a few patients who had had kidney stones before starting bowel tolerance doses who have subsequently had no more difficulty with them. Acute and chronic urinary tract infections are often eliminated; this fact may remove one of the causes of kidney stones. Six patients have had mild pain on urination; five of these patients were over fifty and none had stones.Dr. Humphries also comments about it:
Kidney stones are a theoretical possibility yet have never been shown to be a true risk in the use of vitamin C.Apart from the G6PD deficiency, I don't think probable that a short course of intravenous vitamin C is going to drive any long-term harm when they have not been observed in any of the patients of Dr. Hoffer's study, some of them more than 6 cycles, each cycle 4 weeks, each week three treatments of 0.6g/kg. It is going to be even less likely when using either regular oral or liposomal encapsulated vitamin C.
As a matter of fact an overdose on regular oral vitamin C is near impossible. From wikipedia (my bolds):
Vitamin C is water-soluble, with dietary excesses not absorbed, and excesses in the blood rapidly excreted in the urine. It exhibits remarkably low toxicity. The LD50 (the dose that will kill 50% of a population) in rats is generally accepted to be 11.9 grams per kilogram of body weight when given by forced gavage (orally). The mechanism of death from such doses (1.2% of body weight, or 0.84 kg for a 70 kg human) is unknown, but may be more mechanical than chemical.[103]It seems they die due to electrolytes loss. You may die of too much water ingestion too.
Human Evidence on Intravenous|Intramuscular Vitamin C
There is already enough evidence pointing toward a deficiency of vitamin C under critical illness. Long et alter* write in the abstract of Ascorbic Acid Dynamics in the Seriously Ill and Injured from 2003 (my bolds, TPN = total parenteral nutrition, that is, intravenously delivered, SEM = standard error of the mean):Materials and Methods. Ascorbic acid levels were determined in 12 critically injured patients and 2 patients with severe surgical infections. Each patient received TPN supplemented with increasing doses of ascorbic acid over a 6-day period. Therapeutic responses were determined by plasma and urine measurements using high-pressure liquid chromatography.This advice is acknowledge by Dr. Berger (PN = parenteral nutrition, that is, intravenously delivered) in his 2009 paper:
Results. The initial mean ± SEM baseline plasma ascorbic acid concentration was depressed (0.11 ± 0.03mg/dl) and unresponsive following 2 days on 300 mg/day supplementation (0.14 ± 0.03; P = 1.0) and only approached low normal plasma levels following 2 days on 1000 mg/day (0.32 ± 0.08; P = 0.36). A significant increase was noted following 2 days on 3000 mg/day (1.2 ± 0.03; P = 0.005).
Conclusion. We confirmed extremely low plasma levels of ascorbic acid following trauma and infection. Maximal early repletion of this vitamin requires rapid pool filling early in the post-injury period using supraphysiologic doses for 3 or more days.
It has repeatedly been shown that shocked surgical, trauma, and septic patients have a drastic reduction of circulating plasma ascorbate concentrations. These low concentrations require 3-g doses/d to restore normal plasma ascorbate concentrations.10The problem is that in Long et alter* 3 out of 10 patients were lower than 1.1 mg/dl of vitamin C in plasma (0.34, 0.45, 0.53) after at least two days at 3g/day of intravenous vitamin C. Actually these three received 300mg two days, 1g the next two days and 3g the last two days. Moreover, 2 out of these 10 patients received 3g/day from the beginning and during 6 days. They got their vitamin C urine excretion measured too. Long et alter* wrote (my bolds):
Baseline plasma levels were below normal values for both patients but were increased to normal levels after 1 day on 3000 mg/day. In spite of the high plasma levels on these megadoses for 5 days, it appeared that the ascorbic acid body pools were not filled in 1 or 2 days, as not all the ascorbic acid infused at 3000 mg/day was excreted until the fourth and fifth days.I hope you see the stupidity of the expensive urine argument. I see no reason for decreasing the vitamin C dose without checking blood plasma levels first. Least of all decreasing it in less than 5 days.
From these data it should not be any surprise at all when we find positive results at doses of at least 3g/day for at least five days. What I find quite shocking is the reticence toward its use in gram doses in every and each one of the ICUs around the world given its very low toxicity.
I will review those data I have found about intravenous|intramuscular vitamin C interventions both in non randomized and randomized trials.
Non randomized interventions
Late Dr. Klenner used vitamin C to treat his poliomyelitis patients as he describes in The Treatment of Poliomyelitis and Other Virus Diseases with Vitamin C. Since it is not clear both how many cases had bulbar involvement nor how many of Dr. Klenner's patients had a previous tonsillectomy (I presume less than under other physicians' care), I don't think any inference may be done about vitamin C efficiency in this case. I feel more bold in another case commented upon by Dr. Klenner.So. First a more or less controlled case series by Dr. Klenner. Second an ICU intervention at Vanderbilt hospital.
1.- Acute Viral Haemorrhagic Encephalitis at Annie Penn Memorial Hospital
Dr. Klenner wrote about seven cases admittted to his hospital from 1950 until 1957 in his paper An ‘Insidious’ Virus. All of them were younger than 4 years old (I think that nullifies the tonsillectomy issue concern) and shared similar symptoms described too at his expanding paper The Clinical Evaluation and Treatment of a Deadly Syndrome Caused by an Insidious Virus:Two important stages are recognized in this deadly syndrome: Stage (A)—(1) There is a history of having had “the flu” which lasted forty-eight to ninety-six hours, complicated by extreme physical or mental stress; (2) a mild cold, similar to an allergic rhinitis, which lingered on for several weeks but did not incapacitate the individual. Stage (B)—This stage, which is always sudden, will present itself in at least six forms: (1) convulsive seizure; (2) extreme excitability resembling delirium tremens if an adult and with dancing of the eyeballs if a child; (3) severe chill; (4) strangling in the course of normal eating or drinking; (5) collapse; (6) stupor.He treated 2 of those 7 patients and they survived. The other 5 were attended by other physicians who didn't start any treatment until a diagnosis were made but all of them died before that. If we suppose that Dr. Klenner's clinical judgment was correct then we may estimate the probability of these results being brought by sheer chance alone: (2/7)2∙(5/7)5 = 1.5%.
Perhaps Dr. Klenner judgment was incorrect but these data would at least spark curiosity in any scientific mind.
2.- Vanderbilt University Medical Center Study
This study was based on a new protocol intervention implemented at the ICU of this trauma hospital. It compares the results of the first year of the protocol with a retrospective cohort of the previous year.They cite the paper by Long et alter* and abide to its minimum dose but use a longer duration. From the paper Impact of High-Dose Antioxidants on Outcomes in Acutely Injured Patients by Dr. Collier et alter* from 2008 (my bolds, AO = antioxidants, OR = odds ratio):
Beginning October 1, 2005, we implemented a high dose AO protocol for acutely injured patients. The protocol consisted of ascorbic acid (Hospira, Lake Forest, IL) 1000 mg intravenously in 100 mL NS every 8 hours, α -tocopherol (dl-α-tocopherol acetate, PCCA, Houston, TX) 1,000 IU (1 mL) via naso- or orogastric tube every 8 hours, and selenium (selenious acid, American Regent, Shirley, NY) 200 μg intravenously in 100 mL NS once daily. Ascorbic acid was administered as a bolus over 1 hour (0600-1400-2200 time schedule) and selenium as a bolus over 2 hours (1000 time schedule), although both were permitted to be changed to an enteral dosage form once enteral access was established. The course of treatment was 7 days or until hospital discharge, whichever came first.
The unadjusted relative risk indicates that patients receiving AO have a 30% less risk of dying during their hospitalization (OR 0.70, 95% confidence interval (CI), 0.56-0.88). After adjusting for age, gender, and probability of survival, AO exposure was associated with an even stronger protective effect (OR 0.32, 95% CI 0.22-0.46).
Expected Survivors had a probability of survival >50% (TRISS >0.50) and Expected Deaths had a probability of survival of <50% (TRISS <0.50). For the Expected Survivors group, there appeared to be no benefit to AO treatment (OR 1.0, 95% CI 0.70-1.4, P = .98), whereas the treatment effect was even greater in the Expected Deaths group (OR 0.24, 95% CI 0.15-0.37, P < .001), adjusted and unadjusted.There has been a letter from Dr. Hemilä pointing out an error in the decimal point placement for several number needed to treat (NNT) given elsewhere in the article and confirmed by the authors. The overall unadjusted NNT is 42.7. The adjusted one is 17.10. Both the adjusted and unadjusted NNT for the Expected Deaths group should be 2.13, that is, the estimated relative risk ratio (RR) is 0.47 in this group.
The Expected Deaths group happening to get a better result is from a post hoc analysis. Nevertheless it is important to look for a plausible reason other than pure chance since we are not looking at a non effective intervention we must repartition creatively in order to let it look positive, it was already a positive one on the whole population. I think we have a reason for the higher effectiveness on the more seriously wounded patients:
Intravenously delivered vitamin C and selenium were switched to orally delivered ones (enteral) whenever possible. All the mortality benefit has come to happen only in the Expected Deaths group so it seems that the number of days on parenteral nutrition when analyzing the outcomes should be taken into account, moreover when we have seen that blood concentrations of vitamin C is going to be quite different for intravenous route with respect to oral one.
Randomized trials
I will proceed from the paper by Miller & Hill selecting those two cited with intravenously delivered doses of vitamin C of at least 1g/day and adding the only one after its publication that I am aware of.1.- Cohort of critically ill surgical patients
Dr. Nathens et alter*, Randomized, Prospective Trial of Antioxidant Supplementation in Critically Ill Surgical Patients. Not blinded. Treatment included 1g of vitamin C every 8 hours for the shorter of ICU stay duration or 28 days. They comment on the Results Section:
Five hundred ninety-five patients were enrolled and analyzed, 91% of whom were victims of trauma. The relative risk of pulmonary morbidity was 0.81 (95% confidence interval 0.60–1.1) in patients receiving antioxidant supplementation. Multiple organ failure was significantly less likely to occur in patients receiving antioxidants than in patients receiving standard care, with a relative risk of 0.43 (95% confidence interval 0.19–0.96). Patients randomized to antioxidant supplementation also had a shorter duration of mechanical ventilation and length of ICU stay.This trial was focused on pulmonary morbidity and excluded those highly likely to die as stated in the Protocol Section:
Patients with isolated or severe (Glasgow Coma Scale score = 6 or less) head injury, brain death, anticipated survival less than 48 hours, burns over more than 20% body surface area, sickle cell anemia, need for anticoagulation with coumadin while in the ICU, and chronic renal failure (creatinine > 2.5 mg/dL) were excluded from the trial.There were 5 deaths of 301 patients under treatment versus 9 deaths of 294 patients under standard care. Nathens et alter* comment:
The relative risk of death in the treatment group was 0.55 (95% CI 0.17–1.88). Similar benefits were also evident when ICU mortality and hospital mortality were examined.
One sided-like probability of vitamin C (at the same time that standard care) treatment giving rise to this or better (worse) result by chance alone is 7.4%.
They also measured average plasma concentration of vitamin C in a subset of patients.
2.- Cohort of critically ill cardiac surgery, major trauma, and subarachnoid hemorrhage patients
Berger et alter*, Influence of early antioxidant supplements on clinical evolution and organ function in critically ill cardiac surgery, major trauma, and subarachnoid hemorrhage patients. Blinded. Treatment included 2.7g of vitamin C the first two days, 1.1g day for another rest 3 days (plus 500mg of vitamin C per day as standard care in ICU stay). Why on earth did they cut so short the 2.7+0.5=3.2g/day intervention? Berger et alter* say:Vitamin C is strongly depressed in critical illness, and doses of 3 g per day are required to normalize plasma concentrations [47].This reference is the paper by Long et alter*. This is depressing. As I have pointed out before 3g for two days let 3 out of 10 people with low plasma levels of vitamin C. They are confusing the mean value with the population distribution. Moreover, with such a short high dose duration most of them aren't going to get replenished their body pools either. There is no reason whatsoever for such a short intervention when they were aware of the results obtained by Dr. Nathens et alter* too, their reference 38. I haven't been able to find a justification to use a shorter and lower dose vitamin C intervention. I would bet they didn't read fully the paper by Long et alter*. They seem not to have measures plasma vitamin C concentration.
There were 14 deaths of 102 patients under treatment versus 9 deaths of 94 patients under standard care (RR 1.43). One sided-like probability of vitamin C (at the same time that placebo) treatment giving rise to this or worse (better) result by chance alone is 8.4%.
They acknowledge "more severe brain injuries in the AOX group" (the intervention one) too.
So. I hope any competent mind grasp why these negative intervention doesn't falsify any other one with longer intervention at 3g/day of intravenous vitamin C.
3.- Phase I study in patients with severe sepsis
Fowler et alter*, Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. Blinded. Treatment included three groups:Twenty-four patients with severe sepsis in the medical intensive care unit were randomized 1:1:1 to receive intravenous infusions every six hours for four days of ascorbic acid: Lo-AscA (50 mg/kg/24 h, n = 8), or Hi-AscA (200 mg/kg/24 h, n = 8), or Placebo (5% dextrose/water, n = 8).Lo-AscA would be 3g/day for a 60kg person. Intervention lasted 4 days. Near enough.
28-days-mortality was 62.5% under placebo, 38.1% under Lo-AscA and 50.6% under Hi-AscA. One sided-like probability of vitamin C (at the same time that standard care) treatment giving rise to this or better (worse) result by chance alone is 14.6%.
Foster et alter* comment also:
High dose ascorbic acid patients exhibited significantly faster declines in the regression slopes of delta daily total SOFA scores over time compared to placebo (-0.043 vs. 0.003, p < 0.01) (Figure 2). Placebo patients exhibited a gradual rise in SOFA scores. Though the cohort size is limited, these data suggest that ascorbic acid infusion significantly attenuates the systemic organ injury associated with sepsis.This study has been done by the same Virginia Commonwealth University group studying the impact of ascorbic acid on inhibition of Neutrophil Extracellular Trap (NET) formation that I have already commented upon elsewhere. NET formation seems to be the last salvo when fighting viruses like that of influenza A. Inhibiting it with vitamin C seems to give rise to positive results.
Now, the good news: the Phase II trial is coming.
N=1 (or several) Testing
Late Dr. Klenner explains how he discovered the viricidal and antipyretic action of injectable vitamin C in his paper from 1953:Our interest with vitamin C against the virus organism began ten years ago in a modest rural home. Here a patient who was receiving symptomatic treatment for virus pneumonia had suddenly developed cynosis. He refused hospitalization for supportive oxygen therapy. X-Ray had not been considered because of its dubious value and because the nearest department equipped to give such treatment was 69 miles distant. Two grams of vitamin C was given intramuscularly with the hope that the anaerobic condition existing in the tissues would be relieved by the catalytic action of vitamin C acting as a gas transport aiding cellular respiration. This was an old idea, the important factor being that it worked. Within 30 minutes after giving the drug (which was carried in my medical bag for the treatment of diarrhea in children) the characteristic breathing and slate-like color had cleared. Returning six hours later, at eight in the evening, the patient was found sitting over the edge of his bed enjoying a late dinner. Strangely enough his fever was three degrees less than it was at 2 P.M. that same afternoon. This sudden change in the condition of the patient led us to suspect that vitamin C was playing a role of far greater significance than that of a simple respiratory catalyst. A second injection of one gram of vitamin was administered, by the same route, on this visit and then subsequently at six hour intervals for the next three days. This patient was clinically well after 36 hours of chemotherapy.
Of course the antipyretic and antihistaminic parts are easy to check when you are ill with a simple discontinuation|rechallenge strategy using either Dr. Cathcart's titrate technique with regular vitamin C or Dr. Levy's approach with 4-6g of liposomal vitamin C. There are those of us who are ready to try it in our children too. I translate my friend Ana's experience with her 17 months old son's fever convulsions using vitamin C mixed in juice:
I started with 250mg when he had 38.4°C. We repeated the same dose every 25 minutes until his bowels "roared" loud and clear. He had saturated with 1000mg. The fever was at 37°C and decreased in some meager 5 minutes to 36.4°C.His son has suffered fever convulsions only once more since then when on travel having forgotten to carry vitamin C.
Apart of antihistaminic and antipyretic effect, can we ascertain its antiviral and antibacterial effect pointed out by Drs. Klenner and Cathcart? We have no means of discerning if it has a viricidal or bactericidal effect or not. It may happen that all of the tonsillitis, colds and flu-like illnesses I|we have passed using only vitamin C (without symptoms once saturated) were to last just as long without any treatment at all. Since the longest of them was 5 days I am quite certain they didn't last longer. It's been 5 years without any other antipyretic or antibiotic. It's been 5 years without any time off sick.
At this point it is a credibility issue. As far as I|we have been able to check both late Drs. Klenner and Cathcart predicted the results I|we get. I|we will continue to act as if they are correct while waiting for their hypothesis to be falsified.
Conclusion
It seems to me highly unlikely that ascorbic acid at high doses is completely useless under stresses like either viral or bacterial infections on humans when we have plenty of evidence pointing toward a general positive effect on vertebrates. Is it scientifically proven? Not yet. Neither I have found a refutation of promising preliminary evidence like either the guinea pigs one or the scant humans trials. Given its ridiculous toxicity a simple risk|benefit analysis should drive anyone to act.Of course you can abide to Evidence Based Medicine criteria and put enough blinders on till you don't see any evidence left. Your choice. Good luck.
*[Added on 11/7/2023] I am not fond at all of abbreviations and acronyms. I have been trying to use the complete form of et al. but I was using a complete incorrect one as Athaic has pointed out to me recently. Since it is not clear to me if the neutral one (et alia) is correct in every case or not (there are the masculine et alii and femenine et aliae too) I will surrender and will use the abbreviation until I am certain. One inconvenience still persisting will be that it should not be read aloud without using the complete form or translating it simultaneously to English.













